Abstract
Introduction.Sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) is a potentially life-threatening complication of hematopoietic stem cell transplantation (HSCT). It is characterized by endothelial cell (EC) damage and hepatocellular injury due to chemo- and radio-therapy toxicity, resulting in the local release of pro-inflammatory molecules, cell adhesion activation on endothelial cells and coagulative pathway activation. Fibrinolysis activation produces fibrosis of sinusoids followed by perivascular hepatocyte necrosis and venular occlusion. Defibrotide (DF) consists of a mixture of porcine derived single-stranded phosphodiester oligonucleotides and is efficaciously used in the treatment of SOS/VOD. Various in vivo and in vitro findings demonstrate that DF possesses only minimal anticoagulant activity, with potent antithrombotic and profibrinolytic effects antithrombotic properties, without significant systemic anticoagulant effects. Moreover, DF stimulates sinusoidal endothelium by increasing the availability of proangiogenic factors such as bFGF, protecting them from trypsin digestion. Since the mechanism of action of DF is not completely elucidated, we evaluated its effects on the gene expression of ECs.
Methods.Endothelial colony forming cells (ECFCs) were obtained from umbilical cord blood according to the method of Ingram et al.(Blood 2004;104:2752-2760). Confluent ECFCs at passages III to IV were subjected to 6-h exposure to either Interferon γ (20 ng/ml; 3 experiments) or lipopolysaccharide (100 ng/ml, 4 experiments), with or without Defibrotide (100 ng/ml). Total RNA was extracted and reverted with a RT2 First Strand cDNA Kit (SABiociences, Qiagen). Genotype profile of control and treated cells was evaluated using the Human Endothelial Cell Biology" (PAHS-015Z, SABiociences, Qiagen). Data were analyzed using the RT2 Profiler PCR array data analysis template v3.5 (SABiociences, Qiagen). Relative changes in gene expression were calculated using the Ct (cycle threshold) method.
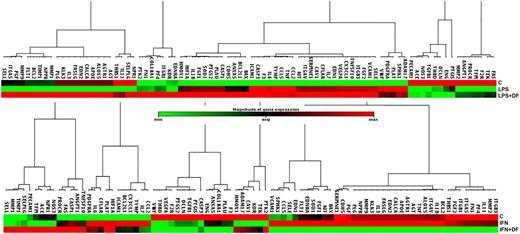
Results. The microarray included 84 genes involved in various pathways, including angiogenesis, cell adhesion, inflammatory response, coagulation and platelet activation, apoptosis, vasoconstriction/vasodilatation. We observed striking different effects of DF depending on the activator used. In particular, LPS treated cells displayed a significant upregulation of several genes, including CCL2, CCL5, CX3CL,TF, IL1B, IL6, endothelial selectin, TNF and VCAM1. DF exposure failed to revert the expression of LPS activated genes at levels comparable with unstimulated cells; nevertheless several genes scarcely expressed in LPS-treated cells were highly expressed in LPS+DF treated cells (Figure 1, upper panel). Similarly, CCL2, CCL5, CX3CL1, endothelial selectin, IL6 and VCAM1 were highly expressed in IFN-γ treated cells, although their expression was significantly lower in IFN-γ+DF treated cells (Figure 1, lower panel).
Conclusions. These findings suggest that DF effects might be highly dependent on the specific activation setting. In particular, LPS and IFN-γ use different cell signaling pathways, though their downstream effects might partially overlap. According to our observations, DF effects could be mediated by the prevalent interference with specific signaling pathways more than by the direct modulation of target genes. This hypothesis agrees with experimental evidences indicating that DF is internalized by EC but dos not enter nuclei (Palomo et al. Blood 2016;127:1719-27). In alternative, the contemporary exposure of cells to DF and LPS, might result in their competition for modulating in opposite directions the same pathways, thus hampering the inhibitory effects of DF. This second hypothesis agrees with the evidence that DF attachment on cell membrane is sufficient to elicit its anti-inflammatory effect ((Palomo et al. Blood 2016;127:1719-27).
Teofili: Jazz Pharmaceutical: Research Funding. Orlando: Jazz Pharmaceutical: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal